Case Study:
Primary VS Established Cell Lines.
What was the challenge?
In vitro models based on human cells are designed and used to generate human data that cannot be obtained in non-human in vivo models that by-pass translational issues, particularly in immunology. The research industry is currently largely dependent on the use of primary human peripheral blood mononuclear cells (PBMCs). Alternative established (cell lines) immune cells are also available. Primary and established human immune cells both have their advantages and disadvantages. The preference of either depends on the experimental design and the nature of query.
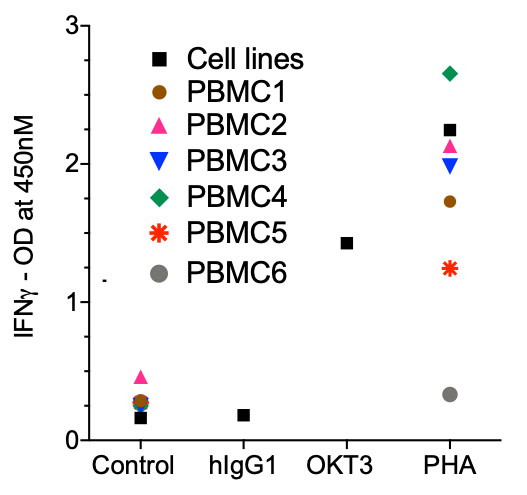
IFNg
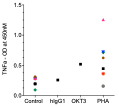
TNFa
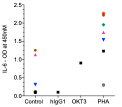
IL-6
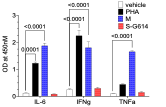
Cell line CRA was stimulated with PHA and M protein or S-G614 protein of SARS-CoV-2. Data was similar to PBMC-based CRA.
Approach:
Immundnz has adapted the standard PBMC-based CRA to a cell line based CRA. A mixed population of myeloid and lymphoid cell lines representing immune cells in PBMCs has been formulated in specific ratios which can be stimulated with anti-CD3 antibody or phytohaemagglutinin (PHA) to secrete cytokines associated with cytokine release syndrome (CRS) or cytokine storm (CS).
Findings:
(i)The cytokine release is consistent and the model can be stimulated with any other mitogens/immunogens.
(ii)Compared to low or high response in PBMC-based CRA, the cytokine levels are average and moderate.
Problem:
To use a cytokine release assay (CRA) that provides a consistent control reference for cytokine secretion when stimulated with a known mitogen. PBMC based CRA is costly, time consuming and can be inconsistent due to donor-dependent variability.
Added Value:
(i)Cells can be customized to facilitate mechanistic studies
(ii)Reproducible